Your Trusted Biocompatibility and Toxicology Expert Services
W&S Consulting provides expert biocompatibility and toxicology solutions for medical device innovators. We accelerate your path to market through rigorous safety assessments, seamless compliance guidance, and strategic support backed by decades of industry experience.

About Us
Global Market Intelligence: Combined Excellence
At W&S, our unique partnership between W&S Consulting Inc. (South Korea) and W&S Toxicology Consulting (India) delivers exceptional value through a seamless integration of local market knowledge and specialized technical expertise.
Our Value Proposition:
Your journey with us begins with personalized local support from our South Korean team. They understand your regional regulatory landscape, business culture, and specific market challenges. This local presence ensures clear communication, responsive service, and tailored solutions aligned with your needs.
Behind this local support stands our Indian team of board-certified toxicologists, who provide world-class technical expertise and comprehensive testing services through our established network of accredited third-party laboratories. This distributed approach allows us to maintain rigorous scientific standards while significantly reducing costs compared to traditional consulting models.
The W&S Advantage:
Cost-effective solutions without compromising on quality or expertise
Local representation and support in South Korea
Access to Board-certified Toxicologists with extensive regulatory experience
Streamlined testing services through vetted laboratory partnerships
Comprehensive understanding of international regulatory requirements
Faster turnaround times through our optimized workflow
Our Services

Biological Evaluation Plan & Biological Evaluation Report
Our strategic approach to medical device biological evaluation integrates device categorization, risk assessment, and testing rationales into customized Biological Evaluation Plans (BEP). We transform complex biological data into clear, scientifically-justified Biological Evaluation Reports (BER) that strengthen your regulatory submissions and validate device safety. Our expertise ensures compliance while minimizing unnecessary testing, accelerating your path to market.

Toxicological Risk Assessment of Medical device constituents
Our toxicological risk assessments (TRA) deliver comprehensive evaluations of leachables and extractables per ISO 10993-17, establishing tolerable intake levels (TI) and margin of safety calculations. We transform chemical characterization data into robust safety assessments, supporting your regulatory submissions with scientifically justified safety conclusions for each device constituent.
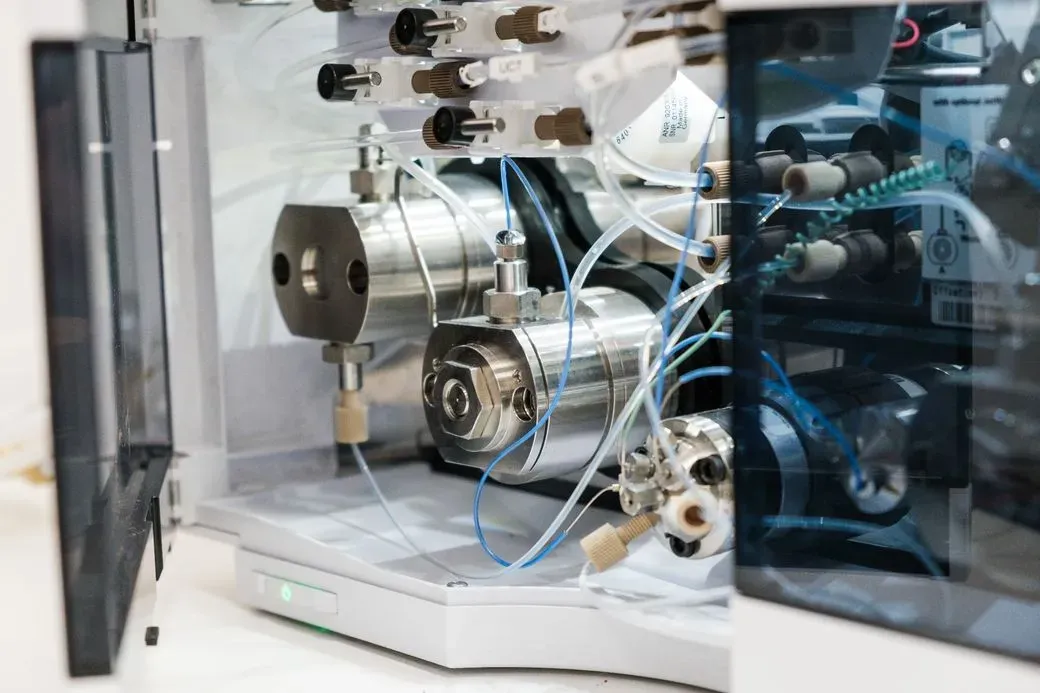
Chemical Characterization of Medical Device Materials
Our service facilitates comprehensive chemical characterization of medical device materials through our partnership with an accredited third-party testing laboratory. This analysis identifies and quantifies chemical substances to ensure regulatory compliance and patient safety.
Our partner laboratory employs advanced analytical instrumentation, including:
Gas and Liquid Chromatography-Mass Spectrometry (GC-MS/MS, LC-MS/MS)
Inductively Coupled Plasma (ICP/MS) techniques for elemental analysis

Biocompatibility testing
We simplify biocompatibility testing through expert laboratory coordination and project management. Our team navigates the complexities of GLP testing requirements, providing seamless access to qualified laboratories while ensuring rigorous oversight, quality execution, and timely delivery of the ISO 10993 testing program.

Study Monitoring
W&S Consulting also specialises in study monitoring to ensure quality, compliance, and reliability in toxicological and biocompatibility evaluations. Our experts oversee the testing process to guarantee adherence to protocols and regulatory requirements.
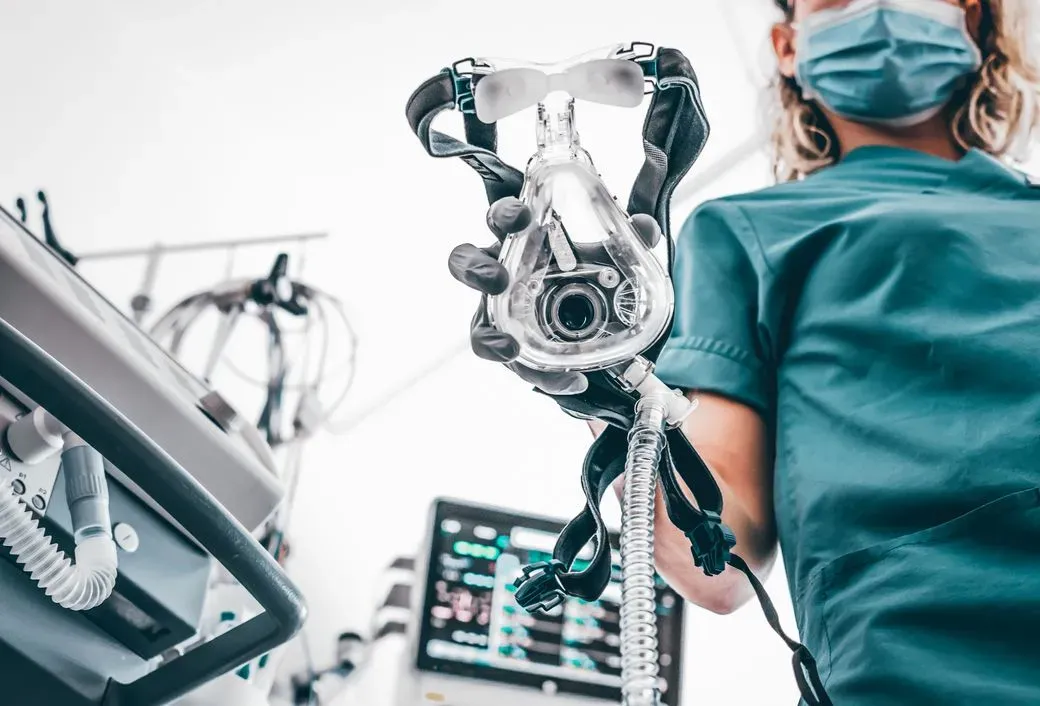
ISO 18562
Specialized evaluations of breathing gas pathways in medical devices, focusing on chemical emissions and particulates.
Team

Kiwoong Jang
CEO & Business Development
Kiwoong has over 18 years of experience in the medical device industry and has worked at global companies as a Regulatory Affairs role for 10 years leading to 8 years of experience at UL as medical sales & development lead.
He has led the medical device Safety, EMC, GMA(Global Market Access), Usability testing, Cyber security services and has launched Biocompatibility and Biological evaluation services with Hansoo Lee.

Dr Sairam Kishore Arava
Principal Toxicologist & Director
Dr. Sairam is an American Board-Certified Toxicologist (DABT) and European Registered Toxicologist with over 20 years of experience. Previously a Senior Toxicologist at Underwriters Laboratory, he managed global medical device projects, including orthopaedic, gas pathways, dental, coronary implants, and 3D-printed devices.
His expertise in ISO 10993 standards and toxicological risk assessments has helped clients secure FDA 510(k) and Notified Body clearances. Dr Sairam holds a PhD in Toxicology from the University of Madras and achieved his DABT certification in 2018. His contributions include hundreds of toxicological assessments, Biological evaluation plans and reports.

Hansoo Lee
Sales & Operation Director
Hansoo is an expert at biocompatibility and biological evaluation services with over 11 years of experience in the medical device industry. He has developed and led the biological evaluation services at a global company for over 8 years and has managed thousands of biocompatibility and chemical characterization testing services on hundreds of medical devices.
He has provided numerous training sessions to medical device companies and associations. He holds bachelor’s degrees in biomedical engineering from Yonsei university.